Autoxidation in Feeds
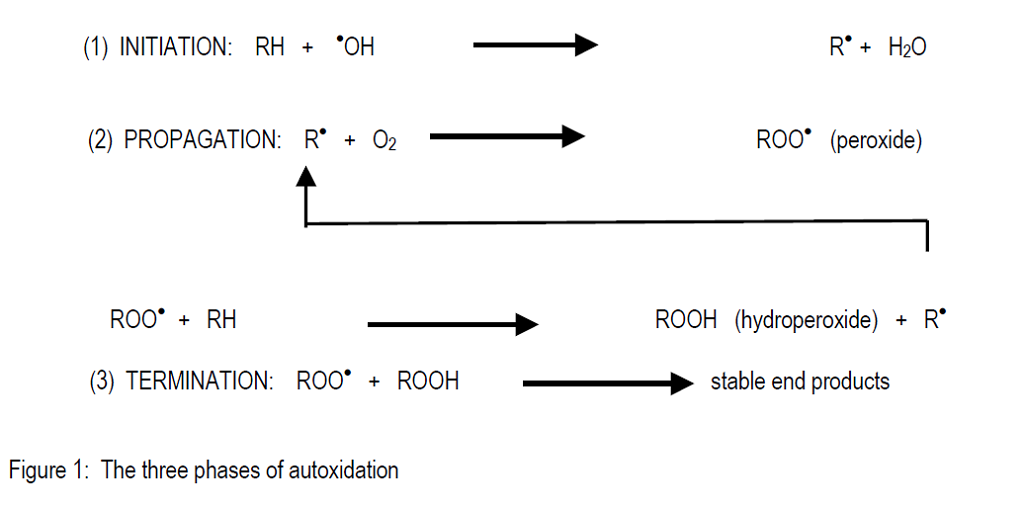
The autoxidation processes are fairly complex but have been reasonably well established and can be described as a free radical chain reaction. There are three separate phases in the overall reaction of autoxidation.
In the first phase, fat oxidation is initiated by various free radicals which are very reactive molecules (Benzie, 1996). In general the oxidisable substrate (RH) is converted into an alkyl radical by proton orelectron abstraction. Metal catalysed production of radicals is by far the most important reaction in this phase. Cobalt, copper, iron and manganese are the most important metals for the initiation of autoxidation. Free radicals may also be generated under the influence of enzymes, light or heat.
The second phase, propagation, occurs when a critical level of free radicals react with oxygen to generate a chain reaction and produce alkyl peroxides. The peroxides react further with new substrate molecules to generate hydroperoxides and more free radicals. The propagation phase is characterised by rapid consumption of oxygen and the generation of heat which in extreme cases can destroy the feed materials and even ignite materials such as fish meal which has a significant oil content.
The third phase, termination, comprises the recombination of various species of free radicals to produce stable end products. These are a wide variety of hydrocarbons, aldehydes, ketones, alcohols and organic acids. This has the effect of slowing down oxidation and it also generates characteristic unpalatable or rancid flavours and odours, which makes feed unacceptable to the animal.